
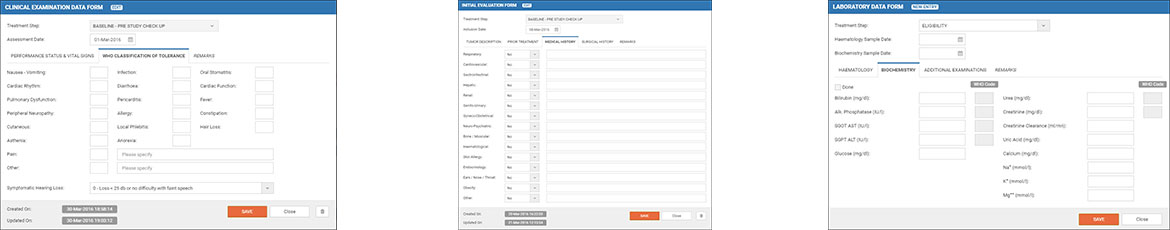
e-CRF is highly specialized and carefully crafted clinical trial platform that provides a high level of flexibility to wide range of trial cases and strategies. It’s a multi-user application that supports various studies on multiple sites. A patient can be enrolled through a very explicit workflow with various eligibility criteria and an automated randomization process is used for distributing the patients over the study’s arms. The e-CRF platform provides many hundreds of fields, regarding Initial Evaluation, Laboratory, Clinical Examination, Treatment, Disease Evaluation, Summary & Follow-Up sections. Lots of indexes, auto-complete and calculated fields are used to speed up the process. In one sentence: no matter how complex or specialized a trial maybe, e-CRF ensures its success.
The all-in-one clinical trials platform and application
Mobile Technology Supported: Web application
Web version of the App: Yes
Cloud Service used: Private Cloud owned by the client
Supported Bandwidth Requirements: 1 TB
Supported concurrent User Requirements: 3.000
[read more=’Read more’ less=’Read less’]
The Development
The main target for this e-CRF platform, is to provide a fast, secure, all-in-one solution for running clinical trials. So, how does one achieve such a goal? First and foremost, by understanding what this “all-in-one” term should include…
Experts combined
We are experts in software development, but we can all work our magic if we have a clear picture of what we have to build. So, we called the doctors! Yes, e-CRF was carefully designed with the help of many well experienced doctors and clinical researchers. With their aid:
- We designed a fully parameterized system that can easily adapt to a variety of clinical studies/trials
- Since it’s mostly a data entry application, extra attention was given to every form just to make sure that is very clear and straight forward to fill-in
- Validation is performed before any data commit, ensuring that no obvious errors or inconsistencies will be tolerated
Mission: Ultimate security
Clinical trials maybe as innocent as test for a headache pills or as serious as cancer treatment strategies. Whatever they are, they affect people’s lives and privacy. So it is evident that date protection and security is the most critical aspect of development for e-CRF. To achieve the highest level of security we implemented to strategies:
- All data are fully encrypted and the user access to the system is secured using a very strict role/permission based administration system.
- Support for further statistical analysis is achieved through the export of depersonalized trial data in the most common formats.
[/read]

